Biointerfaces
Adhesion and forces at biointerphases - From one, to two, to many single molecular interactions
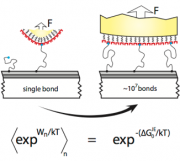 All active systems that are subject to change, motion or flow of matter (i.e. all biological systems, and all mechanical systems) are subject to fundamental forces that drive and steer the way in which atoms, molecules and ultimately macroscopic structures develop, evolve, adapt and age. In essence, dynamic molecular interactions are everything! Consequently, the study of interactive forces is a shared and fundamental interest in seemingly unrelated fields, such as biophysics and adhesion, or corrosion science and stem cell research.
However, we still lack a detailed understanding how macroscopic properties dynamically evolve from molecular forces and interactions; examples include adhesive interactions or adaptive feedback-triggered interactions such as specific and redox-couple interactions.
We showed how it is possible to directly extrapolate a macroscopic work of adhesion based on single molecule Atomic Force Microscopy. We are now using this strategy to extend our understanding of adhesion and interactions at complex biologic interfaces.
All active systems that are subject to change, motion or flow of matter (i.e. all biological systems, and all mechanical systems) are subject to fundamental forces that drive and steer the way in which atoms, molecules and ultimately macroscopic structures develop, evolve, adapt and age. In essence, dynamic molecular interactions are everything! Consequently, the study of interactive forces is a shared and fundamental interest in seemingly unrelated fields, such as biophysics and adhesion, or corrosion science and stem cell research.
However, we still lack a detailed understanding how macroscopic properties dynamically evolve from molecular forces and interactions; examples include adhesive interactions or adaptive feedback-triggered interactions such as specific and redox-couple interactions.
We showed how it is possible to directly extrapolate a macroscopic work of adhesion based on single molecule Atomic Force Microscopy. We are now using this strategy to extend our understanding of adhesion and interactions at complex biologic interfaces.
Hydrophobic interactions, peptides at interfaces and self assembly
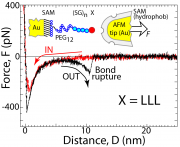 Interactions between hydrophobic moieties steer ubiquitous processes in aqueous media, including the self-organization of biologic matter. Recent decades have seen tremendous progress in understanding these for macroscopic hydrophobic interfaces. Yet, it is still a challenge to experimentally measure hydrophobic interactions at the single-molecule scale and thus to compare with theory.
We develop methods for the experimental characterization of single molecular hydrophobic interactions, and we directly compare our experiments with simulations at the molecular scale. Our main strategy is to examine precisely controllable sequences of interacting end-grafted hydrophobic peptides where interactions can be tuned with exact control using model repeat scaffolds.
Interactions between hydrophobic moieties steer ubiquitous processes in aqueous media, including the self-organization of biologic matter. Recent decades have seen tremendous progress in understanding these for macroscopic hydrophobic interfaces. Yet, it is still a challenge to experimentally measure hydrophobic interactions at the single-molecule scale and thus to compare with theory.
We develop methods for the experimental characterization of single molecular hydrophobic interactions, and we directly compare our experiments with simulations at the molecular scale. Our main strategy is to examine precisely controllable sequences of interacting end-grafted hydrophobic peptides where interactions can be tuned with exact control using model repeat scaffolds.
Materials Interfaces
Degradation and corrosion
 What affects almost every man made modern material used in cars, aircrafts, pipelines and even the implants you may need at some point? Degradation, decay and ultimately complete loss of functionality! We try to understand degradation and we aim to slow it down significantlty.
For instance for metals specifically, corrosion is an often surprisingly fast and unexpected process of inevitable decay. At an estimated cost of 3% of the GDP annually, it carries a steep price tag, not to mention, the potential safety, environmental and health hazards it poses. Our work focusses on understanding nano-to-microscale degradation mechanims and invention of unique real-time analysis methods for vizualizing the initial steps of corrosion of structural and functional metals and metal/polymer composites. Specifically, we are interested in corrosion in confinement, which is crevice corrosion and pitting corrosion: We aim to deliver models and predictions of how and when materials in confined spaces corrode, and how this corrosion can be slowed down.
What affects almost every man made modern material used in cars, aircrafts, pipelines and even the implants you may need at some point? Degradation, decay and ultimately complete loss of functionality! We try to understand degradation and we aim to slow it down significantlty.
For instance for metals specifically, corrosion is an often surprisingly fast and unexpected process of inevitable decay. At an estimated cost of 3% of the GDP annually, it carries a steep price tag, not to mention, the potential safety, environmental and health hazards it poses. Our work focusses on understanding nano-to-microscale degradation mechanims and invention of unique real-time analysis methods for vizualizing the initial steps of corrosion of structural and functional metals and metal/polymer composites. Specifically, we are interested in corrosion in confinement, which is crevice corrosion and pitting corrosion: We aim to deliver models and predictions of how and when materials in confined spaces corrode, and how this corrosion can be slowed down.
Molecular level understanding of solid-liquid interfaces
 Any process at solid/liquid interfaces critically depends on how ion and liquid molecules structure in the inner/compact electric double layer, and on how this influences e.g. interaction sites of adhesion or reactivity (blocking or enhancement). For instance, aqueous electrolytes have long been considered to simply provide the matrix for processes in biological systems and evolution of life. Yet, quite in contrast, at the molecular scale, both water and hydrated ions are, in many cases, essential ingredients that steer molecular processes responsible for structure formation, molecular self-assembly, self-organization of organic matter, and activation of molecular pathways.
Any process at solid/liquid interfaces critically depends on how ion and liquid molecules structure in the inner/compact electric double layer, and on how this influences e.g. interaction sites of adhesion or reactivity (blocking or enhancement). For instance, aqueous electrolytes have long been considered to simply provide the matrix for processes in biological systems and evolution of life. Yet, quite in contrast, at the molecular scale, both water and hydrated ions are, in many cases, essential ingredients that steer molecular processes responsible for structure formation, molecular self-assembly, self-organization of organic matter, and activation of molecular pathways.
In many areas it is often not even clear if, or how, solvent molecules and ions may actively participate in interfacial processes. Typically these electric double layer stuctures are only a few nanometers thick and hence notoriously difficult to study. What are the often dynamically adapting atomic level structures that drive processes at interfaces? Can these be vizualized under dynamic conditions? We aim to achive atomic-resolution 3D imaging of electric double layers (water films and ion-layering) during variation of environmental condition (pH, ions and electrochemical potentials). For achieving this we design new scanning probe instrumentation, and we utilize highly stable atomic force microscopes.
Instrumental Design
The Electrochemical Surface Forces Apparatus
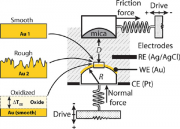 A few years ago we newly designed an electrochemical surface forces apparatus (EC-SFA) that allows control and measurement of surface potentials and interfacial electrochemical reactions with simultaneous measurement of normal interaction forces (with nN resolution), friction forces (with μN resolution), and distances (with Å resolution) between apposing surfaces. The EC-SFA uniquely allows one to look at complex long-term transient effects of dynamic processes (e.g., relaxation times, oxidation reduction cycles, corrosive processes), as well as adhesive and friction forces while tuning electrochemical surface potentials.
A few years ago we newly designed an electrochemical surface forces apparatus (EC-SFA) that allows control and measurement of surface potentials and interfacial electrochemical reactions with simultaneous measurement of normal interaction forces (with nN resolution), friction forces (with μN resolution), and distances (with Å resolution) between apposing surfaces. The EC-SFA uniquely allows one to look at complex long-term transient effects of dynamic processes (e.g., relaxation times, oxidation reduction cycles, corrosive processes), as well as adhesive and friction forces while tuning electrochemical surface potentials.


