This is an old revision of the document!
Oxidation of Catalytically Active Surfaces
In heterogeneous catalysis, e. g., CO oxidation in automotive catalytic converters, the reactants are adsorbed on the surface of the catalyst, where they finally react to form the desired product. Our current line of research mainly deals with oxygen on transition metal surfaces and its reaction with the simple molecules H2 and CO. All these activities are done in close collaboration with several other experimental and computational groups, a combined effort that turned out to be highly productive.
Ultra-thin oxides – a zoo of new phases at surfaces
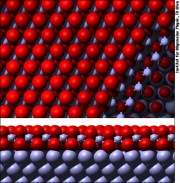 Our group, together with Georg Kresse, was the first one to solve the structure of a purely 2-dimensional oxide, i.e., an oxide that only exists as an ultrathin layer on a metal surface [1]. After this initial discovery, we have found a large variety of further oxides on the Pd(111) surface, allowing us to derive the building rules that govern how these structures are formed [2]. Rh, an immediate neighbor of Pd in the periodic table, forms a so-called tri-layer oxide, i.e., a layer sequence of O-Rh-O on top of the metal [3], and it turned out that this oxide is universal for all the low-index crystallographic orientations of Rh [i.e., (111), (100), (110)] [4].
Most of our work on ultrathin oxides was in close collaboration with Edvin Lundgren, who was in our group when he discovered the 2D oxide on Pd(111), and is now at the Department of Synchrotron Radiation Research of Lund University.
Our group, together with Georg Kresse, was the first one to solve the structure of a purely 2-dimensional oxide, i.e., an oxide that only exists as an ultrathin layer on a metal surface [1]. After this initial discovery, we have found a large variety of further oxides on the Pd(111) surface, allowing us to derive the building rules that govern how these structures are formed [2]. Rh, an immediate neighbor of Pd in the periodic table, forms a so-called tri-layer oxide, i.e., a layer sequence of O-Rh-O on top of the metal [3], and it turned out that this oxide is universal for all the low-index crystallographic orientations of Rh [i.e., (111), (100), (110)] [4].
Most of our work on ultrathin oxides was in close collaboration with Edvin Lundgren, who was in our group when he discovered the 2D oxide on Pd(111), and is now at the Department of Synchrotron Radiation Research of Lund University.
When it comes to reactions, it is still a highly controversial topic whether the surface oxides are extremely good catalysts for oxidation processes or catalytically dead. We could show that the trilayer Rh oxide reacts only at its borders at very low gas pressures [still in the ultrahigh vacuum (UHV) range] [5,6]. This was expected since gases such as H2 and CO do not adsorb on the oxygen-terminated surface of the 2D oxide. We could directly describe the macroscopic reaction kinetics by the data derived form our STM experiments. At elevated temperatures and close to atmospheric pressure, recent results of our collaboration partner in this field, Edvin Lundgren, showed a 1:1 correlation between the existence of a surface oxide and high catalytic activity [7], in contrast to what one should expect from the UHV experiments.
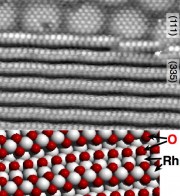 Quite recently, we have gone our way further, and we have identified one-dimensional oxides on stepped surfaces. Formation of these 1D oxides can lead to significant restructuring or roughening of the surface, and 1D oxides can show higher activity of catalytic processes than others form of oxygen bound on surfaces. It was also interesting to discover that 1D oxides impede further 2D oxidation, similar to 2D oxides protecting the underlying metal from further (3D) oxidation.[8]
Quite recently, we have gone our way further, and we have identified one-dimensional oxides on stepped surfaces. Formation of these 1D oxides can lead to significant restructuring or roughening of the surface, and 1D oxides can show higher activity of catalytic processes than others form of oxygen bound on surfaces. It was also interesting to discover that 1D oxides impede further 2D oxidation, similar to 2D oxides protecting the underlying metal from further (3D) oxidation.[8]
We have also discovered the structure of a surface that was probably the first one where the existence of a 2D oxide was suggested: the (4×4) phase of oxygen on Ag(111), again considered an active phase for a catalytic process in industry (ethylene epoxidation). Surprisingly, it turned out that this structure has to be considered metallic silver that only forms grooves to make space for the oxygen.[9] Georg Kresse, who did the ab-initio calculations in this project, found that this structure is stable only due to van-der-Waals forces, quite unusual for a metal surface!
Catalysis on the oxide, not the metal
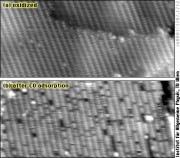 Until the end of the 1990ies, it was believed that reactions like CO oxidation on platinum-group metals always occur by simple adsorption of oxygen on the surface; the O adatoms would then react with adsorbed CO to form CO2, the so-called Langmuir-Hinshelwood mechanism. STM experiments conducted in our group have helped to find out that high catalytic activity can be due oxidation of the catalyst; it was shown that RuO2 formed at sufficient oxygen pressure catalyzes CO oxidation by the so-called Mars-van Krevelen mechanism, where the oxide itself provides oxygen for the reaction.[10] We have continued these studies in collaboration with Herbert Over and his group in Giessen, most recently by examining HCl and Cl on the RuO2(110) surface; with the aim of better understanding the oxidation of HCl, an important industrial process (Deacon process).
Until the end of the 1990ies, it was believed that reactions like CO oxidation on platinum-group metals always occur by simple adsorption of oxygen on the surface; the O adatoms would then react with adsorbed CO to form CO2, the so-called Langmuir-Hinshelwood mechanism. STM experiments conducted in our group have helped to find out that high catalytic activity can be due oxidation of the catalyst; it was shown that RuO2 formed at sufficient oxygen pressure catalyzes CO oxidation by the so-called Mars-van Krevelen mechanism, where the oxide itself provides oxygen for the reaction.[10] We have continued these studies in collaboration with Herbert Over and his group in Giessen, most recently by examining HCl and Cl on the RuO2(110) surface; with the aim of better understanding the oxidation of HCl, an important industrial process (Deacon process).
References
- E. Lundgren, G. Kresse, C. Klein, M. Borg, J.N. Andersen, M. De Santis, Y. Gauthier, C. Konvicka, M. Schmid and P. Varga
Two-dimensional oxide on Pd(111)
Phys. Rev. Lett. 88 (2002) 246103 ⋅ full text - J. Klikovits, E. Napetschnig, M. Schmid, N. Seriani, O. Dubay, G. Kresse, P. Varga
Surface oxides on Pd(111): STM and density functional calculations
Phys. Rev. B 76 (2007) 045405 ⋅ full text - J. Gustafson, A. Mikkelsen, M. Borg, E. Lundgren, L. Köhler, G. Kresse, M. Schmid, P. Varga, J. Yuhara, X. Torrelles, C. Quirós, J.N. Andersen
Self-limited growth of a thin oxide layer on Rh(111)
Phys. Rev. Lett. 92 (2004) 126102 ⋅ full text - E. Lundgren, A. Mikkelsen, J.N. Andersen, G. Kresse, M. Schmid, P. Varga
Surface oxides on close-packed surfaces of late transition metals
J. Phys. Cond. Matter 18 (2006) R481-R499 ⋅ full text* - J. Klikovits, M. Schmid, J. Gustafson, A. Mikkelsen, A. Resta, E. Lundgren, J.N. Andersen, and P. Varga
Kinetics of the reduction of the Rh(111) surface oxide: Linking spectroscopy and atomic-scale information
J. Phys. Chem. B 110 (2006) 9966-9975 ⋅ full text* - E. Lundgren, J. Gustafson, A. Resta, J. Weissenrieder, A. Mikkelsen, J.N. Andersen, L. Köhler, G. Kresse, J. Klikovits, A. Biedermann, M. Schmid, P. Varga
The surface oxide as a source of oxygen on Rh(111)
J. Electron Spectrosc. Relat. Phen. 144-147 (2005) 367-372 ⋅ full text* - J. Gustafson, R. Westerström, A. Resta, A. Mikkelsen, J. N. Andersen, O. Balmes, X. Torrelles, M. Schmid, P. Varga, B. Hammer, G. Kresse, C. Baddeley, E. Lundgren
Structure and catalytic reactivity of Rh oxides
Catalysis Today 145 (2009) 227-235 ⋅ full text* - J. Klikovits, M. Schmid, L.R. Merte, P. Varga, R. Westerström, A. Resta, J. N. Andersen, J. Gustafson, A. Mikkelsen, E. Lundgren, F. Mittendorfer, G. Kresse
Step-orientation-dependent oxidation: from 1D to 2D oxides
Phys. Rev. Lett. 101 (2008) 266104 ⋅ full text - M. Schmid, A. Reicho, A. Stierle, I. Costina, J. Klikovits, P. Kostelnik, O. Dubay, G. Kresse, J. Gustafson, E. Lundgren, J.N. Andersen, H. Dosch, P. Varga
Structure of Ag(111)-p(4×4)-O: no silver oxide
Phys. Rev. Lett. 96 (2006) 146102 ⋅ full text - H. Over, Y.D. Kim, A.P. Seitsonen, S. Wendt, E. Lundgren, M. Schmid, P. Varga, A. Morgante, G. Ertl
Atomic-scale structure and catalytic reactivity of the RuO2(110) surface
Science 287 (2000) 1474-1476 ⋅ full text*
* Please note: access to full text (PDF files) of some articles is restricted due to copyright reasons.


