This is an old revision of the document!
Research Topics
Oxidation of catalytically active surfaces
In heterogeneous catalysis, e. g., CO oxidation in automotive catalytic converters, the reactants are adsorbed on the surface of the catalyst, where they finally react to form the desired product. Our current line of research mainly deals with oxygen on transition metal surfaces and its reaction with the simple molecules H2 and CO. All these activities are done in close collaboration with several other experimental and computational groups, a combined effort that turned out to be highly productive.
Ultra-thin oxides – a zoo of new phases at surfaces
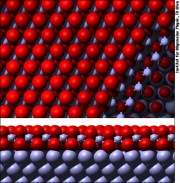 Our group, together with Georg Kresse, was the first one to solve the structure of a purely 2-dimensional oxide, i.e., an oxide that only exists as an ultrathin layer on a metal surface [1]. After this initial discovery, we have found a large variety of further oxides on the Pd(111) surface, allowing us to derive the building rules that govern how these structures are formed [2]. Rh, an immediate neighbor of Pd in the periodic table, forms a so-called tri-layer oxide, i.e., a layer sequence of O-Rh-O on top of the metal [3], and it turned out that this oxide is universal for all the low-index crystallographic orientations of Rh [i.e., (111), (100), (110)] [4].
Most of our work on ultrathin oxides was in close collaboration with Edvin Lundgren, who was in our group when he discovered the 2D oxide on Pd(111), and is now at the Department of Synchrotron Radiation Research of Lund University.
Our group, together with Georg Kresse, was the first one to solve the structure of a purely 2-dimensional oxide, i.e., an oxide that only exists as an ultrathin layer on a metal surface [1]. After this initial discovery, we have found a large variety of further oxides on the Pd(111) surface, allowing us to derive the building rules that govern how these structures are formed [2]. Rh, an immediate neighbor of Pd in the periodic table, forms a so-called tri-layer oxide, i.e., a layer sequence of O-Rh-O on top of the metal [3], and it turned out that this oxide is universal for all the low-index crystallographic orientations of Rh [i.e., (111), (100), (110)] [4].
Most of our work on ultrathin oxides was in close collaboration with Edvin Lundgren, who was in our group when he discovered the 2D oxide on Pd(111), and is now at the Department of Synchrotron Radiation Research of Lund University.
When it comes to reactions, it is still a highly controversial topic whether the surface oxides are extremely good catalysts for oxidation processes or catalytically dead. We could show that the trilayer Rh oxide reacts only at its borders at very low gas pressures [still in the ultrahigh vacuum (UHV) range] [5,6]. This was expected since gases such as H2 and CO do not adsorb on the oxygen-terminated surface of the 2D oxide. We could directly describe the macroscopic reaction kinetics by the data derived form our STM experiments. At elevated temperatures and close to atmospheric pressure, recent results of our collaboration partner in this field, Edvin Lundgren, showed a 1:1 correlation between the existence of a surface oxide and high catalytic activity [7], in contrast to what one should expect from the UHV experiments.
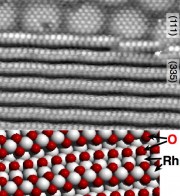 Quite recently, we have gone our way further, and we have identified one-dimensional oxides on stepped surfaces. Formation of these 1D oxides can lead to significant restructuring or roughening of the surface, and 1D oxides can show higher activity of catalytic processes than others form of oxygen bound on surfaces. It was also interesting to discover that 1D oxides impede further 2D oxidation, similar to 2D oxides protecting the underlying metal from further (3D) oxidation.
Quite recently, we have gone our way further, and we have identified one-dimensional oxides on stepped surfaces. Formation of these 1D oxides can lead to significant restructuring or roughening of the surface, and 1D oxides can show higher activity of catalytic processes than others form of oxygen bound on surfaces. It was also interesting to discover that 1D oxides impede further 2D oxidation, similar to 2D oxides protecting the underlying metal from further (3D) oxidation.
We have also discovered the structure of a surface that was probably the first one where the existence of a 2D oxide was suggested: the (4×4) phase of oxygen on Ag(111), again considered an active phase for a catalytic process in industry (ethylene epoxidation). Surprisingly, it turned out that this structure has to be considered metallic silver that only forms grooves to make space for the oxygen. Georg Kresse, who did the ab-initio calculations in this project, found that this structure is stable only due to van-der-Waals forces, quite unusual for a metal surface!
Catalysis on the oxide, not the metal
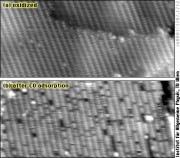 Until the end of the 1990ies, it was believed that reactions like CO oxidation on platinum-group metals always occur by simple adsorption of oxygen on the surface; the O adatoms would then react with adsorbed CO to form CO2, the so-called Langmuir-Hinshelwood mechanism. STM experiments conducted in our group have helped to find out that high catalytic activity can be due oxidation of the catalyst; it was shown that RuO2 formed at sufficient oxygen pressure catalyzes CO oxidation by the so-called Mars-van Krevelen mechanism, where the oxide itself provides oxygen for the reaction. We have continued these studies in collaboration with Herbert Over and his group in Giessen, most recently by examining HCl and Cl on the RuO2(110) surface; with the aim of better understanding the oxidation of HCl, an important industrial process (Deacon process).
Until the end of the 1990ies, it was believed that reactions like CO oxidation on platinum-group metals always occur by simple adsorption of oxygen on the surface; the O adatoms would then react with adsorbed CO to form CO2, the so-called Langmuir-Hinshelwood mechanism. STM experiments conducted in our group have helped to find out that high catalytic activity can be due oxidation of the catalyst; it was shown that RuO2 formed at sufficient oxygen pressure catalyzes CO oxidation by the so-called Mars-van Krevelen mechanism, where the oxide itself provides oxygen for the reaction. We have continued these studies in collaboration with Herbert Over and his group in Giessen, most recently by examining HCl and Cl on the RuO2(110) surface; with the aim of better understanding the oxidation of HCl, an important industrial process (Deacon process).
The Complex World of Aluminum Oxides
 Initial oxidation of pure or almost-pure aluminum leads to rather disordered oxides, with varying thickness, currently not accessible for rigorous examination of the surface structure. Aluminum alloys are different, especially these that liberate aluminum atoms rather slowly, allowing the oxide to take its time forming a well-ordered structure.
Initial oxidation of pure or almost-pure aluminum leads to rather disordered oxides, with varying thickness, currently not accessible for rigorous examination of the surface structure. Aluminum alloys are different, especially these that liberate aluminum atoms rather slowly, allowing the oxide to take its time forming a well-ordered structure.
The best-known well-ordered alumina (=aluminum oxide) film is that on NiAl(110), discovered in the early 1990ies. After numerous attempts by both experimental and computational groups, we finally obtained high-resolution STM images of this surface in 2004, which provided the basis for ab-initio calculations by Georg Kresse, finally solving the old puzzle. It turned out that the building rules of the film are different from all bulk alumina phases, and even the stoichiometry is different from Al2O3. This could be understood after detailed analysis – then it all appeared so simple! The Al atoms forming the interface between oxide and substrate cannot supply all their electrons to the oxide, they need to bind to the metal underneath. So their formal charge is not Al3+ but Al2+. To keep the oxide charge-neutral, more Al atoms than in Al2O3 are needed.
We have also studied defects in these ultrathin alumina films, as well as an alumina film on a Cu-Al alloy that turned out to have exactly the same structure as the one on NiAl(110). Obviously, this structure is a very stable configuration.
Filling a hole
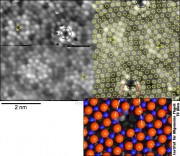 A surprise came when we looked at the surface oxide on another alloy surface, Ni3Al(111). Again, we obtained atomic resolution, and with the experience obtained in the meanwhile we thought that the structural model was quite clear. Georg Kresse again did ab-inito calculations, again stressing his computer cluster to the limit (the cell has >1200 atoms!). Our simple model did not show the correct symmetry seen in the STM images. So Georg suggested that there must be a hole in the oxide at the corner of the unit cell, and suddenly everything was fitting nicely!
A surprise came when we looked at the surface oxide on another alloy surface, Ni3Al(111). Again, we obtained atomic resolution, and with the experience obtained in the meanwhile we thought that the structural model was quite clear. Georg Kresse again did ab-inito calculations, again stressing his computer cluster to the limit (the cell has >1200 atoms!). Our simple model did not show the correct symmetry seen in the STM images. So Georg suggested that there must be a hole in the oxide at the corner of the unit cell, and suddenly everything was fitting nicely!
The funny aspect was yet to discover: With a diameter of about 0.4 nm (0.0000004 mm), these holes are just wide enough to fill in a few atoms, which sit in the hole roughly one on top of the other. We need 3 palladium atoms to fill the hole; then the slightly protruding uppermost Pd atom provides a nucleus for growing larger metal clusters. Thus, we have discovered a template for growing well-ordered metal clusters regularily spaced by 4.1 nm – one of the nicest self-organized template surfaces in nanotechnology so far!
The downside – when we tried to measure the magnetic properties of small Co and Fe clusters grown on this substrate, which would have been highly interesting, it turned out that the substrate is slightly ferromagnetic below a temperature of ≈ 100 K, while we would have to cool the clusters to lower temperatures to see them become ferromagnetic.
Ultrathin metal films
Although growth of ultrathin metal films is generally considered a mature subfield of surface science, surprises are still possible. That was the case about a decade ago when we discovered that ferromagnetic iron films thought to have face-centered cubic structure are actually body-centered cubic (See the Crystallography of Iron Films page of our STM Gallery. As we currently have a detailed look at surface diffusion of metal atoms, we find strange things again. Expect to read more about this soon.
Writing magnetic nanostructures by ion irradiation
 In the upcoming age of nanotechnology, one can easily imagine that demand will arise for various magnetic structures in the sub-micrometer range. Creating these structures by conventional lithography is a complicated and thus costly process. Irradiation by a focused ion beam, or an ion beam patterning system such as currently under development at IMS Nanofabrication AG should provide a one-step alternative.
In the upcoming age of nanotechnology, one can easily imagine that demand will arise for various magnetic structures in the sub-micrometer range. Creating these structures by conventional lithography is a complicated and thus costly process. Irradiation by a focused ion beam, or an ion beam patterning system such as currently under development at IMS Nanofabrication AG should provide a one-step alternative.
Iron films grown on Cu(100) are in a metastable nonmagnetic fcc state between about 1 – 2 nm thickness, or more, if suitable additives are used. It was one of the many clever ideas of Albert Biedermann, then postdoc in our group (now at the University of Vienna) to initiate the transformation into the stable ferromagnetic bcc state by ion irradiation. We have verified this by magneto-optic Kerr-effect (MOKE) measurements. Using an ion beam patterner at IMS, we could exploit this phenomenon to write magnetic structures with a resolution of ≈100 nm, probably limited by the resolution of the magnetic force microscope used to image them as well as by the non-perfect focus reached on our comparably rough sample.
Pulsed laser deposition – growth by energetic particles
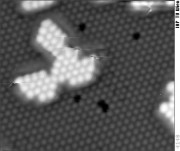 Pulsed laser deposition (PLD) is a simple, yet powerful method to grow thin films of a large variety of materials, and it was widely studied in the applied physics community after the success of growing high-temperature superconductors PLD in the 1980ies. It is known since more than two decades that the particles ablated by by short laser pulses have high kinetic energy (dozens to hundreds of electron volts); when they impinge on the substrate this leads to differences in the morphology and structure of the films as compared to thermal evaporation. Nevertheless, the basic physics of the ablation process by short laser pulses and the processes on the substrate are insufficiently understood.
Pulsed laser deposition (PLD) is a simple, yet powerful method to grow thin films of a large variety of materials, and it was widely studied in the applied physics community after the success of growing high-temperature superconductors PLD in the 1980ies. It is known since more than two decades that the particles ablated by by short laser pulses have high kinetic energy (dozens to hundreds of electron volts); when they impinge on the substrate this leads to differences in the morphology and structure of the films as compared to thermal evaporation. Nevertheless, the basic physics of the ablation process by short laser pulses and the processes on the substrate are insufficiently understood.
We have combined a time-of-flight spectrometer for determination of the particles' energy with high-resolution STM to study the surface structure at the substrate. It turned out that moderate particle energies are sufficient for implantation of ablated particles into the substrate, while higher kinetic energies are required for the creation of additional nuclei that modify the island density. As long as these additional nuclei are not formed, our results nicely fit calculations by classic nucleation theory, taking the time structure of the pulsed deposition into account.
References
- E. Lundgren, G. Kresse, C. Klein, M. Borg, J.N. Andersen, M. De Santis, Y. Gauthier, C. Konvicka, M. Schmid and P. Varga
Two-dimensional oxide on Pd(111)
Phys. Rev. Lett. 88 (2002) 246103 ⋅ full text - J. Klikovits, E. Napetschnig, M. Schmid, N. Seriani, O. Dubay, G. Kresse, P. Varga
Surface oxides on Pd(111): STM and density functional calculations
Phys. Rev. B 76 (2007) 045405 ⋅ full text - J. Gustafson, A. Mikkelsen, M. Borg, E. Lundgren, L. Köhler, G. Kresse, M. Schmid, P. Varga, J. Yuhara, X. Torrelles, C. Quirós, J.N. Andersen
Self-limited growth of a thin oxide layer on Rh(111)
Phys. Rev. Lett. 92 (2004) 126102 ⋅ full text - E. Lundgren, A. Mikkelsen, J.N. Andersen, G. Kresse, M. Schmid, P. Varga
Surface oxides on close-packed surfaces of late transition metals
J. Phys. Cond. Matter 18 (2006) R481-R499 ⋅ full text* - J. Klikovits, M. Schmid, J. Gustafson, A. Mikkelsen, A. Resta, E. Lundgren, J.N. Andersen, and P. Varga
Kinetics of the reduction of the Rh(111) surface oxide: Linking spectroscopy and atomic-scale information
J. Phys. Chem. B 110 (2006) 9966-9975 ⋅ full text* - E. Lundgren, J. Gustafson, A. Resta, J. Weissenrieder, A. Mikkelsen, J.N. Andersen, L. Köhler, G. Kresse, J. Klikovits, A. Biedermann, M. Schmid, P. Varga
The surface oxide as a source of oxygen on Rh(111)
J. Electron Spectrosc. Relat. Phen. 144-147 (2005) 367-372 ⋅ full text* - J. Gustafson, R. Westerström, A. Resta, A. Mikkelsen, J. N. Andersen, O. Balmes, X. Torrelles, M. Schmid, P. Varga, B. Hammer, G. Kresse, C. Baddeley, E. Lundgren
Structure and catalytic reactivity of Rh oxides
Catalysis Today 145 (2009) 227-235 ⋅ full text*
* Please note: access to full text (PDF files) of some articles is restricted due to copyright reasons.


