This is an old revision of the document!
The Complex World of Aluminum Oxides
 Initial oxidation of pure or almost-pure aluminum leads to rather disordered oxides, with varying thickness, currently not accessible for rigorous examination of the surface structure. Aluminum alloys are different, especially these that liberate aluminum atoms rather slowly, allowing the oxide to take its time forming a well-ordered structure.
Initial oxidation of pure or almost-pure aluminum leads to rather disordered oxides, with varying thickness, currently not accessible for rigorous examination of the surface structure. Aluminum alloys are different, especially these that liberate aluminum atoms rather slowly, allowing the oxide to take its time forming a well-ordered structure.
The best-known well-ordered alumina (=aluminum oxide) film is that on NiAl(110), discovered in the early 1990ies. After numerous attempts by both experimental and computational groups, we finally obtained high-resolution STM images of this surface in 2004, which provided the basis for ab-initio calculations by Georg Kresse, finally solving the old puzzle. It turned out that the building rules of the film are different from all bulk alumina phases, and even the stoichiometry is different from Al2O3. This could be understood after detailed analysis – then it all appeared so simple! The Al atoms forming the interface between oxide and substrate cannot supply all their electrons to the oxide, they need to bind to the metal underneath. So their formal charge is not Al3+ but Al2+. To keep the oxide charge-neutral, more Al atoms than in Al2O3 are needed.
We have also studied defects in these ultrathin alumina films, as well as an alumina film on a Cu-Al alloy that turned out to have exactly the same structure as the one on NiAl(110). Obviously, this structure is a very stable configuration.
Filling a hole
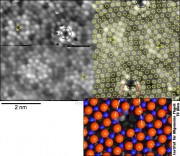 A surprise came when we looked at the surface oxide on another alloy surface, Ni3Al(111). Again, we obtained atomic resolution, and with the experience obtained in the meanwhile we thought that the structural model was quite clear. Georg Kresse again did ab-inito calculations, again stressing his computer cluster to the limit (the cell has >1200 atoms!). Our simple model did not show the correct symmetry seen in the STM images. So Georg suggested that there must be a hole in the oxide at the corner of the unit cell, and suddenly everything was fitting nicely!
A surprise came when we looked at the surface oxide on another alloy surface, Ni3Al(111). Again, we obtained atomic resolution, and with the experience obtained in the meanwhile we thought that the structural model was quite clear. Georg Kresse again did ab-inito calculations, again stressing his computer cluster to the limit (the cell has >1200 atoms!). Our simple model did not show the correct symmetry seen in the STM images. So Georg suggested that there must be a hole in the oxide at the corner of the unit cell, and suddenly everything was fitting nicely!
The funny aspect was yet to discover: With a diameter of about 0.4 nm (0.0000004 mm), these holes are just wide enough to fill in a few atoms, which sit in the hole roughly one on top of the other. We need 3 palladium atoms to fill the hole; then the slightly protruding uppermost Pd atom provides a nucleus for growing larger metal clusters. Thus, we have discovered a template for growing well-ordered metal clusters regularly spaced by 4.1 nm – one of the nicest self-organized template surfaces in nanotechnology so far!
It's not all that simple, however. When we tried to measure the magnetic properties of small Co and Fe clusters grown on this substrate, which are highly interesting, it turned out that the substrate is slightly ferromagnetic below a temperature of ≈ 100 K, while we would have to cool the clusters to lower temperatures to see them become ferromagnetic. Therefore, measurements by our surface magneto-optic Kerr effect setup were not successful and we had to travel to a synchrotron to determine the magnetic properties of these clusters.
References
- G. Kresse, M. Schmid, E. Napetschnig, M. Shishkin, L. Köhler, P. Varga
Structure of the ultrathin aluminum oxide film on NiAl(110)
Science 308 (2005) 1440-1442 ⋅ full text* - M. Schmid, M. Shishkin, G. Kresse, E. Napetschnig, P. Varga, M. Kulawik, N. Nilius, H.-P. Rust, H.-J. Freund
Oxygen-deficient line defects in an ultrathin aluminum oxide film
Phys. Rev. Lett. 97 (2006) 046101 ⋅ full text - E. Napetschnig, M. Schmid, P. Varga
Ultrathin alumina film on Cu-9%Al(111)
Surf. Sci. 602 (2008) 1750-1756 · full text* - M. Schmid, G. Kresse, A. Buchsbaum, E. Napetschnig, S. Gritschneder, M. Reichling, P. Varga
Nanotemplate with holes: Ultra-thin alumina on Ni3Al(111)
Phys. Rev. Lett. 99 (2007) 196104 ⋅ full text - A. Buchsbaum, M. De Santis, H.C.N. Tolentino, M. Schmid, P. Varga
Highly ordered Pd, Fe and Co clusters on alumina on Ni3Al(111)
Phys. Rev. B 81 (2010) 115420 ⋅ full text
* Please note: access to full text (PDF files) of some articles is restricted due to copyright reasons.


