Crystallography of Iron Films
fcc, bcc and the transition
In contrast to most metals, which come in only one crystallographic form, iron (Fe) can have two crystallographic modifications:
- Body centered cubic (bcc) iron, also called alpha (iron or the ferritic phase, and
- face centered cubic (fcc) iron, also called gamma iron or the austenitic phase.
Both structures are built from cubes with atoms at each corner (shown below). The bcc crystal structure has Fe atoms also at the center of each cube, whereas the fcc structure has additional atoms in the sides of each cube.
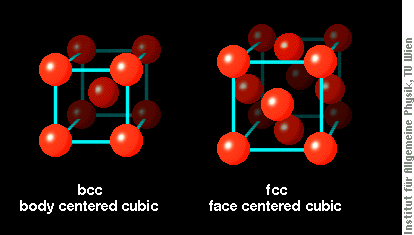
Structure and magnetism
At ambient conditions, pure iron is body-centered cubic (bcc) and ferromagnetic (i.e., it can be magnetized and is strongly attracted by magnets). Above approx. 920 °C, it becomes face centered cubic (fcc). Whereas the bcc phase gains its stability from magnetism (even though it becomes paramagnetic above its Curie temperature of 770 °C), the high-temperature fcc phase is paramagnetic. In order to study the magnetic properties of the fcc phase of pure Fe at lower temperatures, researchers have been experimenting for many decades with metastable fcc Fe precipitates (i.e., inclusions) in copper (Cu) as well as with thin films on an fcc substrate like copper.
In the late 1980s, it was discovered that ultrathin Fe films (less than 5 atomic layers thick) grown on the (100) oriented face of copper single crystals are ferromagnetic, although fcc iron precipitates in Cu are not. Ever since, this observation has been interpreted as direct evidence for the presence of ferromagnetic fcc iron in these films, a hypothetical phase proposed almost 40 years ago (two-gamma state hypothesis by Kaufman, Clougherty and Weiss, Acta Met. 11, 1963).
So let us have a detailed look at the surface of such iron films on Cu(100) now - here is one four atomic layers thick:
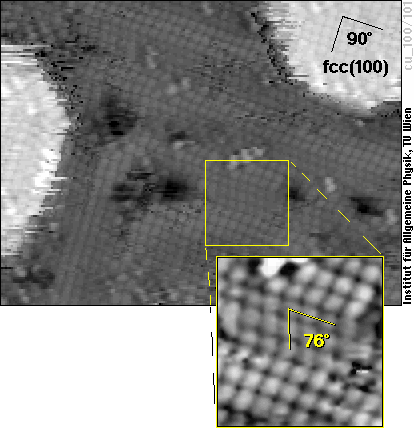
The (100) face of an fcc material is a perfect square lattice, as that one in the upper right part of the image, where the film is five layers thick. What we see in most parts of the image is a zigzag pattern, however. There, the atoms do not form a square lattice (with an angle of 90° between the two directions of atoms), but rather a lattice with approx. 76° angle. By comparing this lattice with that of different crystallographic structures of Fe, it is easy to see that it comes very close to the (110)-surface of bcc iron, much closer than to (100)-oriented fcc iron. The figure below shows schematic views of these two surface structures and also how they relate to the cubes of the bcc and fcc structure shown above.
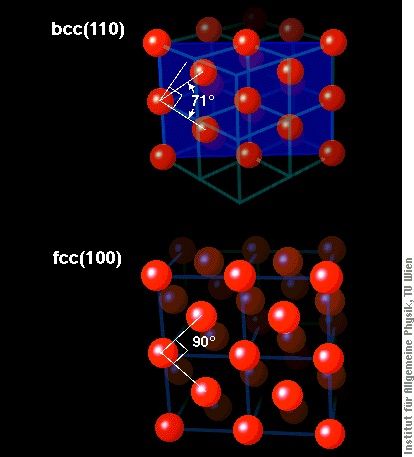
We see such bcc(110)-like structures in films of different thickness between 2.5 and four atomic layers, just the thickness range where they are known to be ferromagnetic. We thus conclude that these ferromagnetic thin Fe films are not fcc, and, hence, do not give any support for the assumption of ferromagnetic fcc iron. Our STM images therefore severely shake the foundations of some basic ideas on the magnetism of iron, a topic that almost everyone would have considered solved by now.
The zig-zag structure observed by us is similar to the microstructure of so called martensitic plates forming in bulk iron when it transforms from its high-temperature fcc phase to bcc upon cooling. Whereas the bcc platelets in the previously known martensitic structures are dozens to hundreds of atoms thick, our zig-zag stripes are just a few atoms wide, i.e., about one nanometer (1 nanometer is one millionth of a millimeter). That's why we call this new phase a nanomartensitic structure.
As the bulk bcc iron phase is directly related to its magnetism, we also believe that the existence of the nanomartensitic structure observed in films just a few atomic layers thick can be explained by the exceptional magnetic properties of such ultrathin films.
The fcc-bcc phase transformation
Interesting enough, iron films on (100)-oriented copper somewhat thicker than those shown above (between approx. five and ten atom layers) are really fcc and, hence, not ferromagnetic. Although bulk iron is bcc at room temperature, it is not unexpected that we find these thin films to consist of fcc iron. The film reaches a lower energy by assuming the slightly unfavorable fcc crystal structure than by forming an interface between the fcc copper substrate and the bcc lattice, where the atoms would have to sit in unfavorable positions.
The real bcc bulk iron phase starts to grow in these fcc films by transformation of small regions (“needles”) mostly eight atoms wide and about a hundred atoms long. Again, we find a surface structure very similar to bcc(110) in these areas:
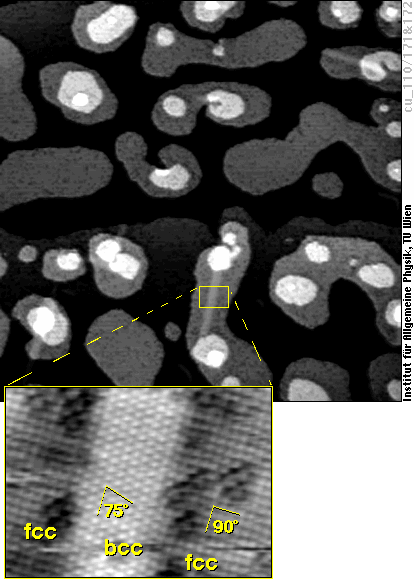
Whereas the structure in this image is a kind of intermediate phase on the way to true bcc iron (it is still distorted to fit the surrounding fcc lattice), these areas finally become true bcc iron. The fcc-bcc phase transformation of iron has immense technological importance, e.g., for the hardening of steel, and now we can study the details of this transformation for the first time atom-by atom!
For further information
- A. Biedermann, R. Tscheließnig, M. Schmid and P. Varga
Crystallographic structure of ultrathin Fe films on Cu(100)
Phys. Rev. Lett. 87 (2001) 086103. Full text
- A. Biedermann, M. Schmid and P. Varga
Nucleation of bcc iron in ultrathin fcc films
Phys. Rev. Lett. 86 (2001) 464-467. Full text
- A. Biedermann, R. Tscheließnig, M. Schmid, P. Varga
Local atomic structure of ultrathin Fe films grown on Cu(100)
Appl. Phys. A 78 (2004) 807-816. Full text*
* Please note: access to full text (PDF files) of some articles is restricted due to copyright reasons.
The one who figured out the physics presented on this page: Albert Biedermann.
Responsible for all the typos etc.: Michael Schmid([email address: lastname @ this server · enable javascript to see it]).


